ANALYSIS OF HAND SANITIZER PRODUCTS USING HANDHELD 1064 nm RAMAN
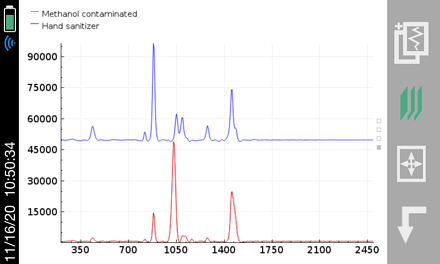
The use of hand sanitizers is becoming part of the everyday norm, and global demand has grown exponentially over the recent months. Hand sanitizers in liquid and gel formulations are being produced by manufacturing companies around the world. But what if these products consumers think are providing protection actually contain hazardous chemicals or fail to provide the protection they are being relied upon for? According to the Centers for Disease Control and Prevention (CDC), it is recommended that consumers use alcohol-based hand sanitizers with at least 60 percent ethanol. However, as the market need has soared at a rapid rate, manufacturers have struggled to keep up with demand. Because of this, products with inadequate levels of alcohol have flooded the marketplace. In addition, the shortage of alcohol-based hand sanitizers has allowed for the introduction of potentially hazardous replacements or products that have an inadequate level of alcohol into the production process. These dangerous chemicals include methanol, 1-propanol, even petroleum, and cause adverse effects on the human body if absorbed through the skin or ingested—including blindness, cardiac issues, effects on the central nervous system and even death.
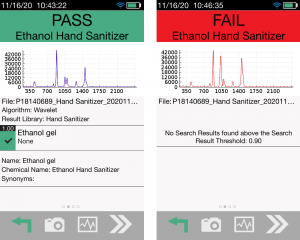
Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), are now monitoring and testing the influx of temporary preparations of hand sanitizers that are manufactured in the U.S., as well as those entering the U.S., by developing guidance documents, issuing import warning lists, and implementing their own testing that utilizes Rigaku handheld Raman technology. There is a global focus on hand sanitizer manufacturing, quality control, and confirmation and the handheld Raman can be a key tool during all stages of quality analysis.
HANDHELD RAMAN FOR THE ANALYSIS OF HAND SANITIZER
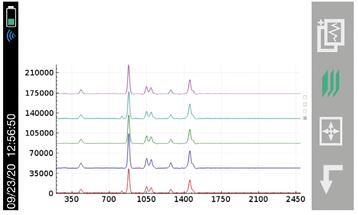
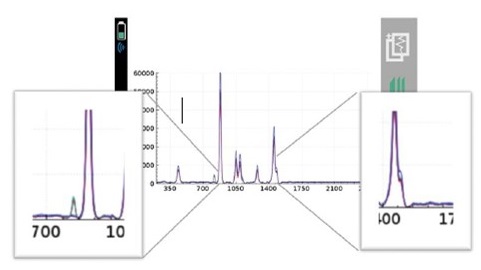
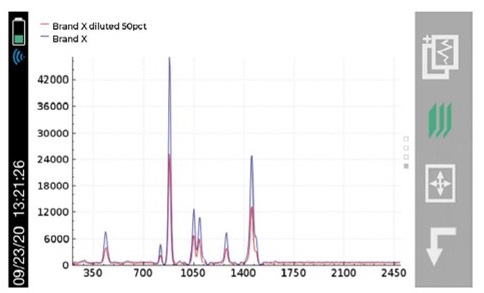
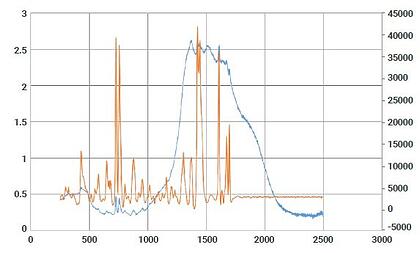
With the recent focus on hand sanitizer production and the potential of hazardous contaminants or sub-par levels of active ingredients, manufacturers and regulatory agencies are in need of a fast and easy analysis method for on-site quality control, confirmation, and a means to protect their brand in anti-counterfeiting measures. The two main active ingredients in most commercially available alcohol-based hand sanitizers are ethyl alcohol (ethanol) and isopropyl alcohol (isopropanol). These chemicals, as well as many possible dangerous contaminants commonly found in handheld sanitizer formulations, are reactive to Raman spectroscopy. Similarly, the fragrances and dyes that are frequently part of this material set are highly favorable to 1064 nm-based Raman provided by the Rigaku Progeny and ResQ portfolio over alternative, lower wavelengths, such as 785 nm or 830 nm. This is due to the fluorescence interference these chemicals are susceptible to when using Raman technology.
CONCLUSION
Consumer demand for hand hygiene products has skyrocketed during the current global pandemic and market reports show there is no end in sight. This growth has brought new manufacturers into the marketspace, as well as opened up a means for criminal activity to capitalize by producing sub-par or dangerous products. To support this “new norm,” regulatory agencies have put processes in place to monitor the import/export process, as well as directly test suspicious shipments. Manufacturers are implementing analysis and testing protocols as part of quality management, as well as a means to protect their brand with anti-counterfeiting measures. The Rigaku Progeny 1064 nm Raman analyzer is the tool of choice among leading regulatory bodies and manufacturers because of its ability to analyze finished products through packaging, as well as identify the chemicals used during manufacturing.